To an atom, that stability comes in the form of an octet, or eight electrons in its valence shell, which provides a stable energy level. How atoms attain an octet is the key. Noble gases, in group VIII, have a natural octet (that’s why they’re so noble). The metals have lesser valence electrons than non-metals and all noble gases have 8 valence electrons except for helium, which has 2 valence electrons. The valence electron of metallic elements depend on their group number and they are having lesser valence electron than non-metals.
Use the group numbers to determine the number of valence electrons. The Group number of a non-transition metal can be used to find the number of valence electrons in an atom of that element. The ones place of the group number is the number of valence electrons in an atom of these elements. A pair of oxygen atoms can form an O 2 molecule in which each atom has a total of eight valence electrons by sharing two pairs of electrons. The term covalent bond is used to describe the bonds in compounds that result from the sharing of one or more pairs of electrons. Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2(n 2) electrons.

Number Of Valence Electrons
The # of valence electrons (VE) is the same as the group #. Valence electrons are the electrons on the valence (or outermost) shell.
Ex:
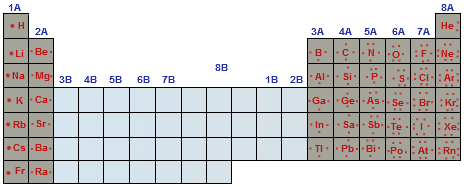
- Group I: 1 VE
- Group II: 2 VE
- Group VI: 6 VE
How To Find Total Valence Electrons


Number Of Valence Electrons In Aluminum
Like most people, atoms seek stability…a secure existence. How people gain stability is by getting an education (yea for you!) and earning an income which helps them to live a better life.
To an atom, that stability comes in the form of an octet, or eight electrons in its valence shell, which provides a stable energy level. How atoms attain an octet is the key. Noble gases, in group VIII, have a natural octet (that’s why they’re so noble). But all other atoms must either gain or lose electrons from their valence shells to form an octet. By knowing the # valence electrons, predictions can be made about the atom’s tendencies.
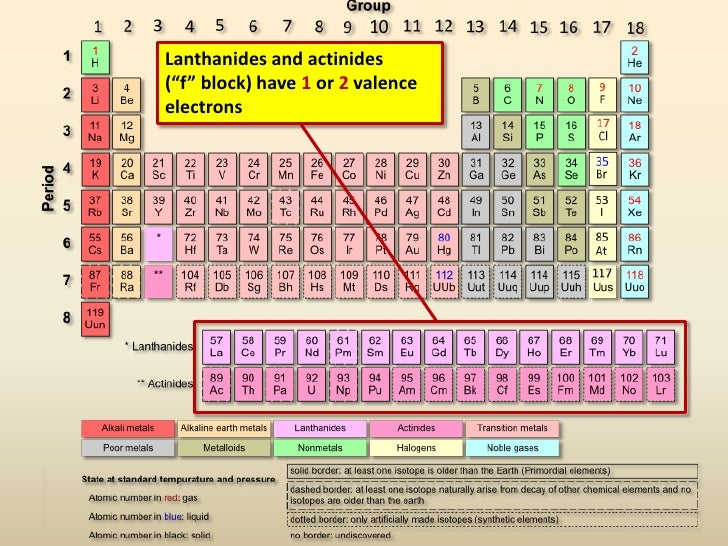
The closer to Group VIII, then the more likely the atom is to gain electrons to progress to the next noble gas and have 8 electrons.
The farther away from Group VIII, then the more likely the atom is to lose electrons to regress to the previous noble gas’ electron configuration to attain 8 valence electrons.
The # valence electrons can give insight into the psychology of an atom and by knowing its needs, one can predict its chemical behavior: what will it want to do to attain an octet and achieve stability.
Periodic trends simply help to explain how the atom thinks and behaves. From that, predictions on bonding and reactivity can be made (rather than mixing a few things and hoping not to scorch your eyebrows).
